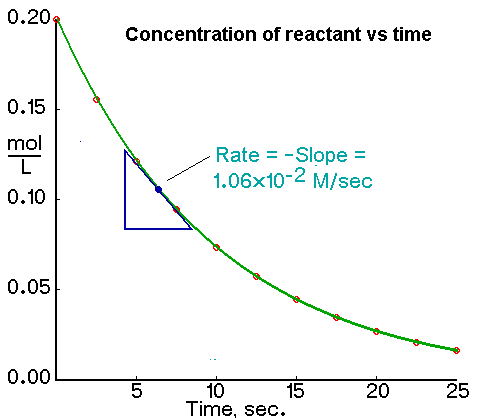
Rate of Reaction Calculation
PPM Calculation Another example of calculating ppm is to use the example of a solution of salt NaCl in water. A n EaR where k A T298n exp-EaRT where T.

How Do I Determine Rate Of Reaction Or Kinetics Of Reaction Without Taking Into Consideration The Reactant Concentration
Document the relevant data including assessments.
. More practical however is to think about corrosion rates in more useful terms such as a corrosion rate in millimeters per year. Group Pile Load Capacity Calculation In order to support heavy loads the piles are arranged in groups. The solution has 0007 grams of salt and the final mass of the solution is 1 kilogram.
All rate constant records for that reaction are returned with a link to Details on that record. For a second order reaction the rate constant has units of liter per mole per second Lmol 1 s 1 or M 1 s 1 For a third order reaction the rate constant has units of liter squared per mole squares per second L 2 mol 2 s 1 or M 2. The first order reaction can be defined as follows.
Include the date and time of the venipuncture. Solving numerical problems Examples about the calculation of the average rate of reaction and instantaneous rate of reaction are shown below. The reaction in which the rate of a reaction is Q.
Calculating Parts Per Million. The startup rate may be positive or negative. For a first order reaction the rate constant has units of per second of s-1.
They then go on to investigate the effect of the addition of a small amount of Mn 2 on the reaction. So we mentioned that 2 ppm 00002 but how would we calculate that. Work out the expression -E A RT and.
The percent ratio of the actual yield to the theoretical yield is known as the percentage yield This Percentage Yield is. Unlike the stoichiometric coefficients determined by calculation the orders of the reaction are based on the kinetics of the reaction. NO 2 CO As an example consider the gas-phase reaction NO 2 CO NO CO 2If this reaction occurred in a single step its reaction rate r would be proportional to the rate of collisions between NO 2 and CO molecules.
Rate of Reaction Calculation. This practical activity takes around 1 hour. Where k is the rate constant or rate coefficient.
There are several methods to determine the modulus of subgrade reaction. The horizontal Soil Subgrade Reaction in Pile Foundations is often determined using the Broms method Broms 1964. Here is how the Rate of chemical reaction calculation can be explained with given input values - 10 500005.
To use this online calculator for Rate of chemical reaction enter Change in concentration ΔC Time Interval Δt and hit the calculate button. Ensure appropriate infusion flow. In this experiment students use a continuous monitoring method to investigate how the rate of the reaction changes with the concentration of MnO 4- ions.
The relationship between reactor power and startup rate is given by the following equation. How to calculate Rate of chemical reaction using this online calculator. Calculate the average rate of reaction in.
This approximation about the rate of a reaction doubling for a 10 degree rise in temperature only works for reactions with activation energies of about 50 kJ mol-1 fairly close to room temperature. 21000000 0000002 100 00002. How is corrosion current used to generate a corrosion rate.
Nt n010 SURt. If you can be bothered use the equation to find out what happens if you increase the temperature from say 1000 K to 1010 K. The above is only an energy conservation equationcon vection and dif-.
Apart from this based on our calculation we calculate the expected yield of a reaction known as the theoretical yield In this article we have discussed the percentage yield formula in detail along with some tips to increase the annual yield. Reactants and if defined reaction products. Record the start of the infusion on the clients chart.
240 cm 3 of carbon dioxide gas is collected. The reaction stops after 15 seconds. Calculation of Corrosion Rate.
In physical chemistry the Arrhenius equation is a formula for the temperature dependence of reaction ratesThe equation was proposed by Svante Arrhenius in 1889 based on the work of Dutch chemist Jacobus Henricus van t Hoff who had noted in 1884 that the van t Hoff equation for the temperature dependence of equilibrium constants suggests such a formula for the. 01 g of calcium carbonate is added to excess hydrochloric acid. Each rate constant record contains the following information as available.
L and m are the orders of the reaction with respect to the reactants A and B respectively. If the time for 80 of a reagent to be consumed initially at a concentration of 10 molL is 25 s. And the sum l m is the overall reaction order.
The orders of the reaction are defined by the m. Do not leave patient care environment until rate is calculated and adjusted accordingly. The maximum horizontal capacity for a given deflection is determined from the modulus of subgrade reaction kNm3.
The gauge and length of the. The numerical result obtained by fitting corrosion data to a model is generally a corrosion current. SUR reactor startup rate dpm decades per minute t time during reactor transient minute The higher the value of SUR the more rapid the change in reactor power.
Its actually pretty simple. Assume an electrolytic dissolution reaction involving a chemical. The method is widely used as it takes into consideration the length of the piles short or long the type of the soil cohesive or cohesionless and the boundary condition at the pile head free-head or fixed-head.
R kNO 2CO where k is the reaction rate constant and square brackets indicate a molar concentrationAnother typical example is the. Rate constant records for a specified reaction are found by searching the Reaction Database. 52 where Q ch is the heat release rate of the chemical reaction and Q R is the heat transfer rate of radiation.
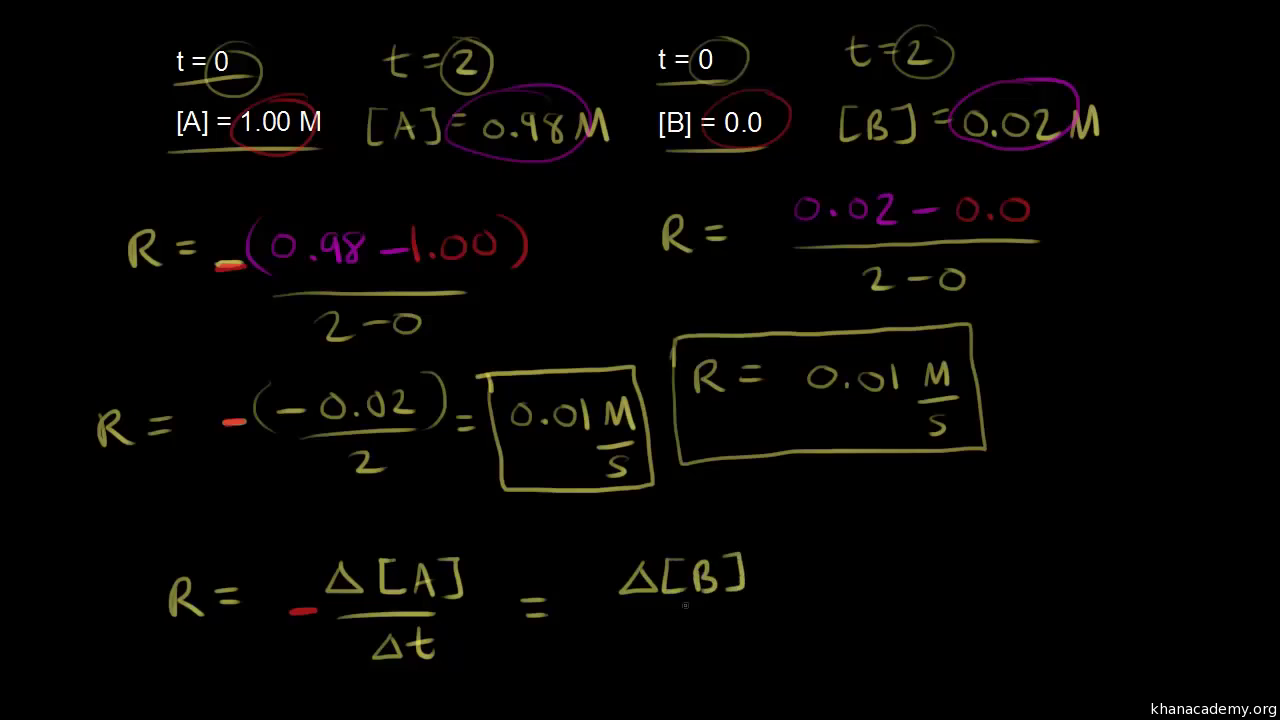
Introduction To Reaction Rates Video Khan Academy

Chemical Equilibrium Reaction Online College Chemistry Courses College Chemistry Online Degree Chemical Kinetics

No comments for "Rate of Reaction Calculation"
Post a Comment